Selective decay resistance of soft
tissue in early land plants (4)
As shown before, the "hollow straw" phenomenon often
observed with
early land plants fossilized in the Rhynie chert requires a
more complex explanation than the one proposed in [1,2]. (See
also Rhynie
Chert News 60,
66,
97.)
It cannot be reduced to a silicification front moving into
the
plant for a short distance only while the larger part of the tissue is
left to decay. Such front would preserve the epidermis first but there
is ample evidence that the epidermis is less well or not at all
preserved in plant parts with hollow straw aspect. The latter is
brought about by a well preserved ring of cortex tissue between the
poorly preserved epidermis and the decayed cortex tissue within.
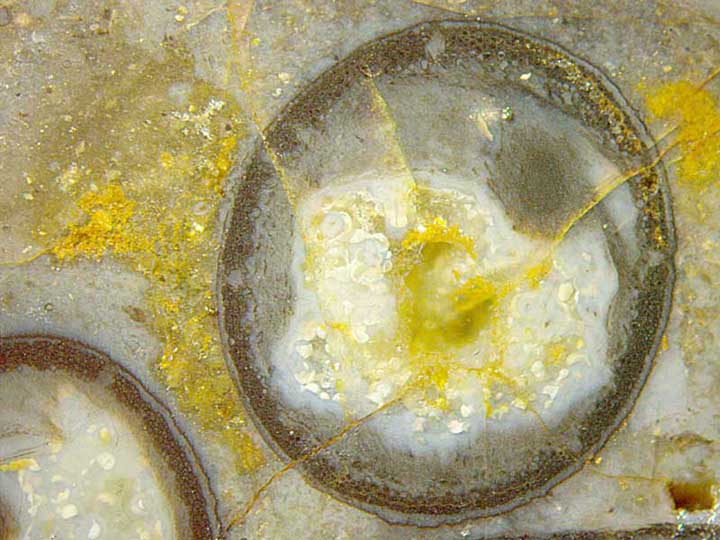
Figs.1,2: Aglaophyton
preserved as hollow straws with enigmatic gaps or chambers in the wall.
Image widths: 6.9mm, 2.8mm.
Fig.3 (below right): Detail of Fig.1: Empty chamber
in the wall of the
straw connected to a gap in the epidermis. Image width: 1.7mm.
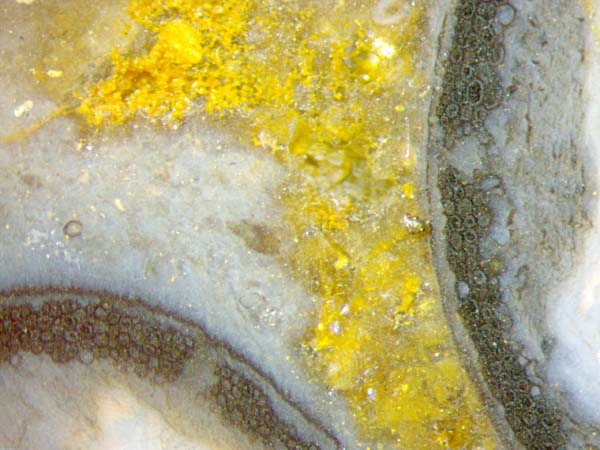
Another
detail supports the idea that the hollow straw aspect is not the result
of diffusion and
silicification but had been purposeful prepared by the living plant: It
is the presence of gaps or chambers in the wall of the straw. There
must be quite a number of them in some straws, judging from 5 of them
seen on the cross-section in Fig.1.
Usually
the cells in the wall of the straw are filled with bluish pale
chalcedony but the dark cell walls make the straw wall appear
dark. The straw in Figs.1-3 is darker than usual because the chalcedony
inside the cells is partially clear so that one looks into the dark
interior. One should be careful not to mistake the dark aspect for a
black fill. Specimens like this one can be
particularly misleading since there
are really black fills in plant cells in other samples of Rhynie chert.
An erronous interpretation is suggested by a few cells in Fig.3 with a
horizontal boundary indicating a deposit within the cell. At first
sight it may look like a dark deposit with whitish chalcedony above but
it is not. It appears that Figs.1-3 are shown here upside down, hence
what had gone on in these cells was silica clusters forming in the
water and settling into a suspension at the bottom which finally turned
into whitish chalcedony while the water above turned into transparent
chalcedony later.
A quite unrelated observation is worth
mentioning here, although it does not contribute to understanding: None
of the various cracks in Figs.1,3 had been deflected along the surface
of the plant where the waxy cuticle on the epidermis usually provides an easy path for crack propagation. It must be concluded that, for reasons
unknown, there had been no easy crack path here.
The
enigmatic chambers in the wall of the straws deserve closer
consideration. There are samples where none of the hollow straws has got chambers of this size. Conspicuous chambers are never seen
on sections
of plants with well-preserved tissue throughout, as in Rhynie
Chert News 2, 85.
Sub-stomatal chambers are so small that they are usually not seen on
sections. Nevertheless they could possibly be the sites where the large
chambers formed later, for whichever reason.
A few more of the large chambers are shown in Rhynie
Chert News 97
and in the pictures below.
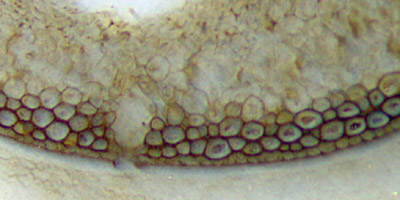

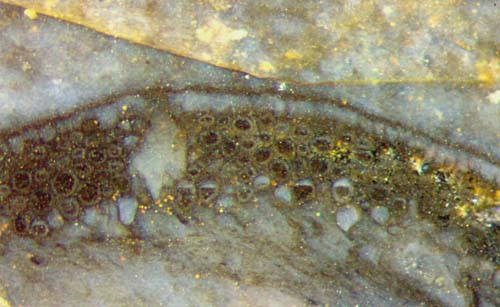
Fig.4 (left): Aglaophyton hollow
straw, chamber in the wall with access
hole in the epidermis and special arrangement of cells .
Fig.5 (right): Aglaophyton
cross-sections, sporangium wall (left) and hollow straw with chamber in
the wall, bulging out at the bottom (right).
Note also the remains of the destroyed epidermis.
A clue to an explanation of the empty chambers or gaps
occasionally present in the wall of hollow
straws may be hidden in Fig.6. It
shows a rare case of
an apparent gap in the dark wall filled with faintly seen cells (Fig.7)
which
have not got the black stain usually present on the cell walls of the
hollow straw. The faintly seen cells fit so neatly to the other ones
that an explanation as newly grown cells filling a hole gnawn by a
creature can be excluded. Hence, the faint cells must have been there
all
the time, and when the living plant prepared a few circumferential
layers of cells by unknown means to become rot resistant in order to
form a persistent tube, apparently several clusters of cells did not
get
that rot resistance and thus were left to decay or dissolved by
themselves, thus leaving holes in the straw. The plant must have done
this on
purpose but it is not known to which purpose. Judging
from Figs.1-4, the epidermis seems to be
involved, which only makes the problem more confusing.
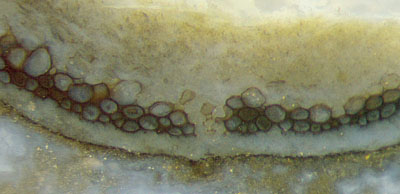
Fig.6 (left): Aglaophyton
hollow straw, epidermis decayed, apparent gap in the dark wall
filled with faintly seen cells, see drawing Fig.7.
Width of
Figs.4,5,6: 1.4mm, 1.7mm, 1.4mm. Equal scale for Figs.3-6.
Fig.7 (below):
Detail of Fig.6 possibly leading the way to a partial explanation of
the intriguing phenomenon of gaps or holes only in those specimens
of
Aglaophyton
which appear as hollow
straws. Width of the drawing 0.4mm.
This drawing ...
... obviously contradicts the assumption in
[1,2] that the
wall thickness represents the depth of silica diffusion,
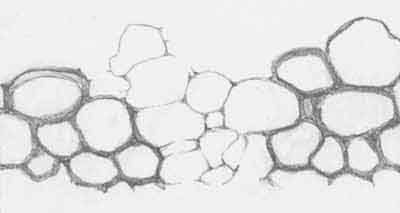 ... shows that a couple of cells is conspicuously
spared from getting a thick dark coating,
... shows that a couple of cells is conspicuously
spared from getting a thick dark coating,
... suggests that the occasional absence of the
dark coating is not incidental but had been controlled by the living plant.
It is not known whether the
cells without protective coating mostly vanished, leaving empty chambers, or persisted as in Fig.6,7. The phenomenon of cell walls vanishing in a
controlled way for a
particular purpose is not quite uncommon among plants. Euphorbias, for
example, make long tubes for poisonous sticky liquid in this way. The same can
be suspected of Nothia. (See Rhynie
Chert News 57.)
The formation of
chambers, gaps, or holes in the wall is all the more peculiar since the
plant would grow a dome-shaped cover
over a hole eaten into the wall so that it would become closed, as seen
in Rhynie
Chert News 60.
Incidentally, the latter
phenomenon indicates once more that the hollow straw aspect did not result from partial silicification of the dead
plant but had been prepared while the plant was alive.
H.-J.
Weiss
2017
2021
[1]
C.L. Powell, N.H. Trewin, D. Edwards: Palaeoecology and
plant succession in a borehole through the Rhynie cherts, ...
Geological Society, London,
Special Publications 180 (2000), 439-457.
[2] www.abdn.ac.uk/rhynie, Chapter Taphonomy.
 |
 |
105 |








 ... shows that a couple of cells is conspicuously
spared from getting a thick dark coating,
... shows that a couple of cells is conspicuously
spared from getting a thick dark coating, 
